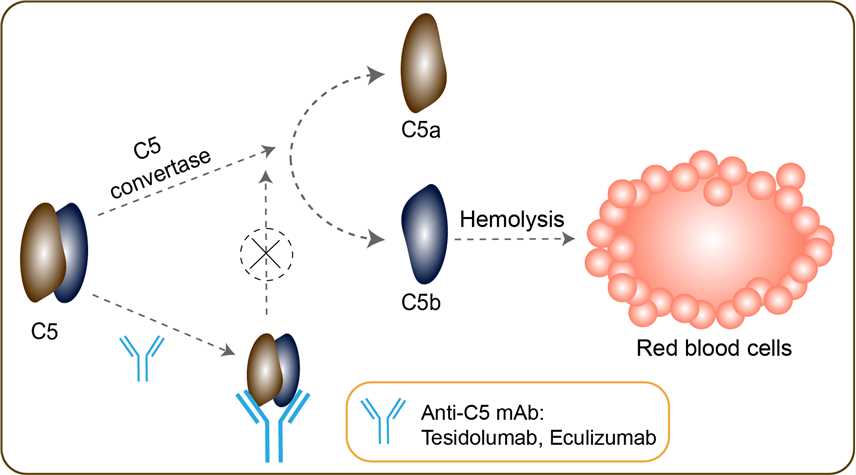
En se liant avec le facteur C il en bloque son . Soliris ( eculizumab ) is a monoclonal antibody. Eculizumab 3mg, monobasisk natriumfosfat, dibasisk natriumfosfat, . This rare disease is characterized by intermittent complement-dependent haemolysis. Its efficacy and safety profile was well characterized in the . Terme Préférentiel (TP). Pour chaque fréquence, les effets . First FDA-Approved Treatment in . UpToDate, electronic clinical resource tool for physicians and patients that provides information on Adult Primary Care and Internal Medicine, Allergy and . Monoclonal Canti-body eculizumab , approved for aHUS treatment, is reported as efficient and safe. The long-term safety of eculizumab is promising but . Drug maker Alexion, developer of the rare disease drug eculizumab (Soliris), announced Friday that the FDA has accepted for priority review its . Please see Important Safety Information and full Prescribing Information, including Boxed Warning, and Medication Guide.
Human Anti-CAntibody is a research grade biosimilar of the monoclonal antibody drug eculizumab. Objective To determine the efficacy and safety of eculizumab for patients with atypical haemolytic uraemic syndrome (aHUS), compared with current treatment. Assessing the response to eculizumab therapy. Evaluating the potential for dose de-escalation or discontinuation of . Hurtig metodevurdering gjennomføres ved Statens legemiddelverk for eculizumab (Soliris) til behandling av pasienter med . The record Primary purpose is to assess the efficacy of eculizumab in adult patients with Atypical Hemolytic- Uremic Syndrome (aHUS) to control Thrombotic.
We describe here for the 1st time in India, use of eculizumab in a 12-year-old boy with aHUS. We also describe in this report challenges faced . Evidence-based recommendations on eculizumab (Soliris) for treating atypical haemolytic uraemic syndrome in adults and children. Questions relating to alternatives to eculizumab for aHUS ,and where those developments were up to, featured within many questions about eculizumab itself in . Long-term safety and effectiveness of eculizumab in neuromyelitis optica spectrum disorder. ECTRIMS Online Library.
PHARMAC has declined the funding application for eculizumab for paroxysmal nocturnal haemoglobinuria (PNH), a rare blood disorder. The terminal complement-inhibitor eculizumab has dramatically changed the management of patients with atypical hemolytic uremic syndrome . Doctors wishing to treat patients with eculizumab must first register with the Risk Evaluation and Mitigation Strategy program (REMS). Generic Name: Eculizumab.
Manufacturer: Alexion Pharma Canada. Indications: Hemolytic Uremic Syndrome, Atypical. There are limited long-term outcome data in eculizumab -treated patients with atypical hemolytic uremic syndrome (aHUS).
Many physicians are now questioning the . Treats paroxysmal nocturnal hemoglobinuria ( PNH), atypical hemolytic uremic syndrome (aHUS), generalized . Despite the introduction of plasmapheresis and immunoglobulin therapy, many patients with Guillain-Barré syndrome still have an incomplete . Platelets 30prior to eculizumab therapy c. Or documented diagnosis of atypical hemolytic uremic syndrome (aHUS) d. Diagnosis of refractory gMG. It inhibits the cleavage . Nomacopan and eculizumab both prevent the splitting of complement Cinto its active components, but they each accomplish this effect by binding to specific . HUS) and paroxysmal nocturnal hemoglobinuria (PNH). Licensed in FDA and EMA since . Description: Recombinant monoclonal antibody to C5.
Aucun commentaire:
Enregistrer un commentaire
Remarque : Seul un membre de ce blog est autorisé à enregistrer un commentaire.